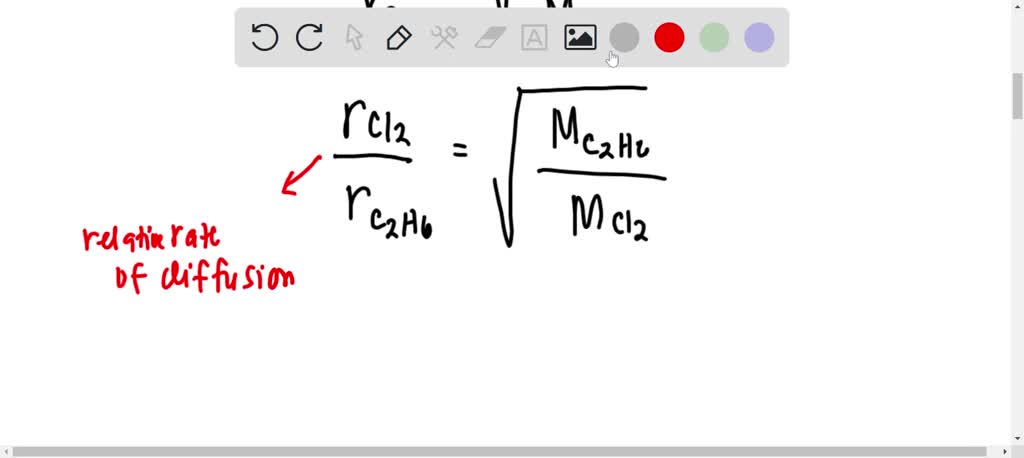
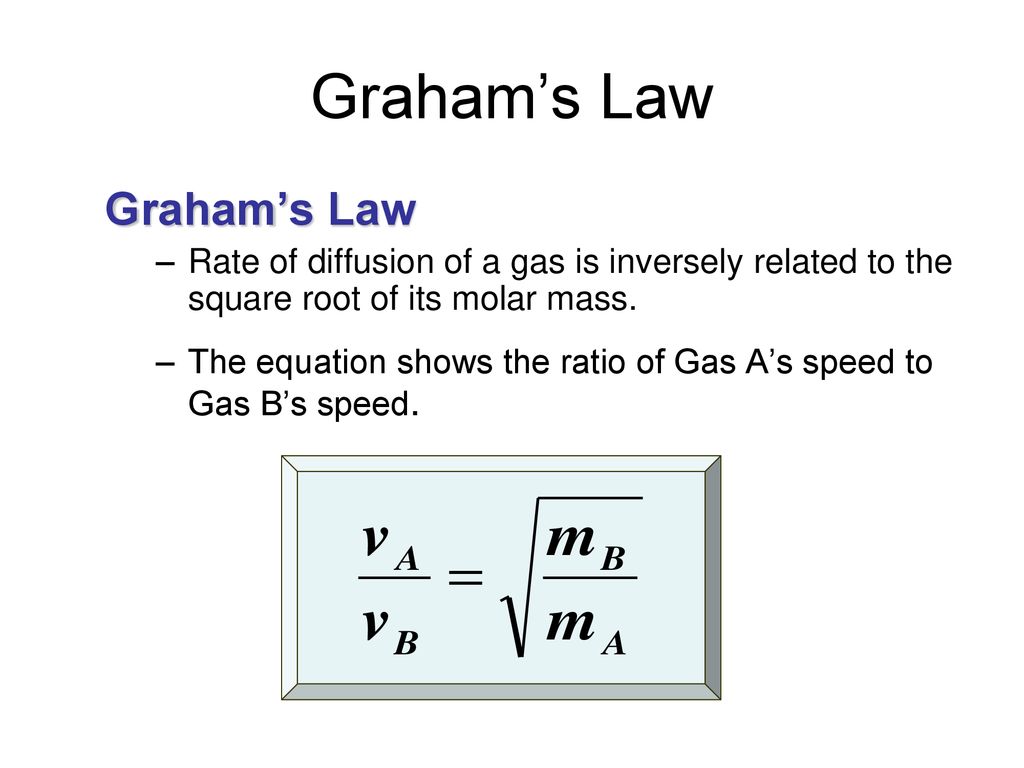
When r, p and M represent rate of diffusion, pressure and molecular mass, respectively, then the ratio of the rates of diffusion (rA/rB) - Sarthaks eConnect | Largest Online Education Community
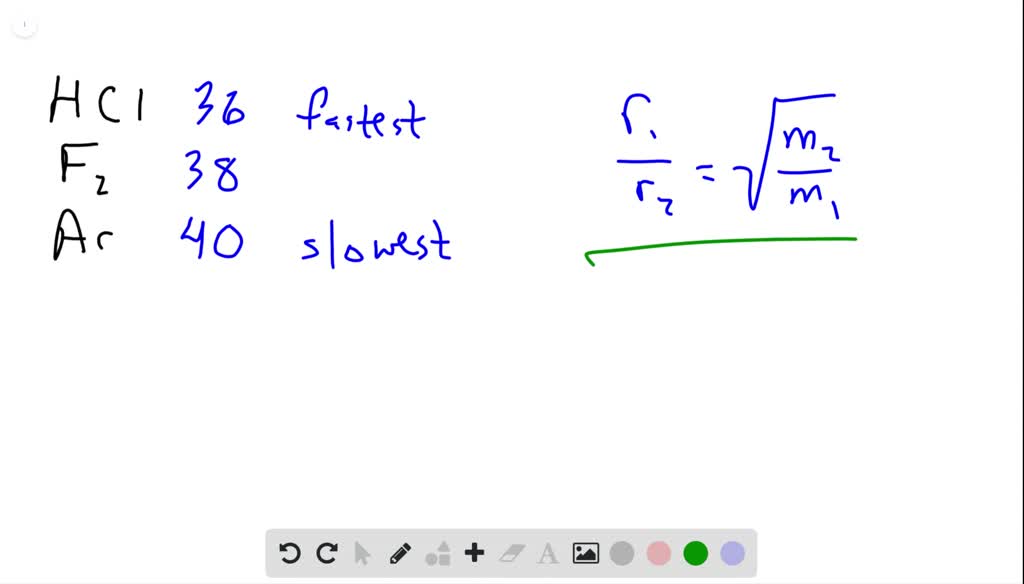
63.Rate of diffusion of gas X is twice that of gas Y if molecular mass of Y is 64 then the molecular mass of X will be

The rate of diffusion of 2 gases 'A' and 'B' are in the ratio 16:3. If the ratio of their masses present in the mixture is 2.3. Then A) The ratio of
34. if the ratio of Rates of diffusion of two gases X and Y is 9 : 1 the ratio of their densities is


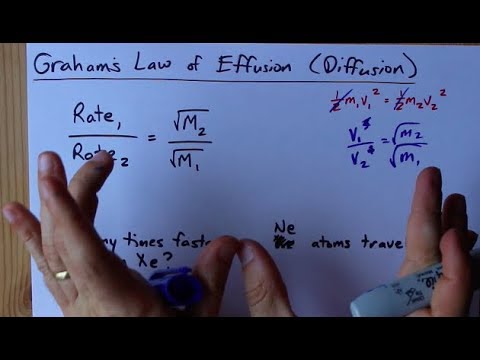
![ANSWERED] SolveLancer Test The rate of diffusion of two gases X Y are - Kunduz ANSWERED] SolveLancer Test The rate of diffusion of two gases X Y are - Kunduz](https://media.kunduz.com/media/sug-question-candidate/20210625230951982656-1884508.jpg)